To develop quantitative MRI methods we need to test the methods on test objects, otherwise known as phantoms. These phantoms are designed to mimic a particular property we expect to observe in the in vivo scans. For example, a phantom may be designed to represent the typical T1 values of the human brain in a 3T MRI scanner.
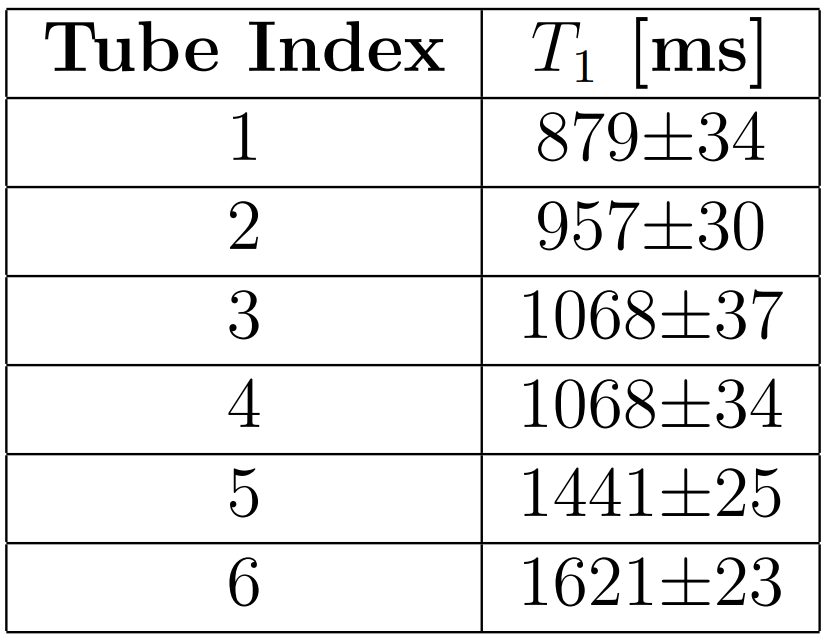
Before testing a new mapping method, the phantom parameters of interest must be characterised. For a custom homemade phantom, the parameters must be measured on-site using a trusted reference scanning method.
For T2, the reference method is often a single-echo spin-echo pulse sequence with long delays between each measurement repetition. The long delays allow full magnetisation relaxation between echo measurements. Please see other posts on this site for more details on reference mapping techniques, such as Implementing a variable echo time method for reference T2-mapping.
Although phantom manufacturers will provide reference parameter values for their phantoms, it is desirable to also characterise the phantom on-site, using the same imaging system to be used for testing the experimental measurement methods. The on-site measurements may then be compared to the manufacturer’s values.
A Phantom for T1 and T2 in the Human Brain
Here we discuss the production of a custom phantom for testing T1 and T2 mapping [Allen2019]. In [Allen2019], a phantom was constructed with T1 and T2 values spanning the range typically observed across grey and white matter in the in vivo human brain at 3T. There is considerable variation in the literature values [Cercignani2018, Bojorquez2017] but approximate ranges for grey matter have been reported as T1 = 1300ms to 1700ms and T2 = 80ms to 120ms. Measured white matter values are around T1 = 800ms to 1000ms and T2= 60ms to 80ms. The custom phantom was used for all sequence development scans in [Allen2019], with the variation of relaxation properties across the phantom allowing exploration of the performance of the relevant mapping techniques.
Structural Design
The internal structure of the phantom was originally designed as part of another study [Brand2016]. It was 3D-printed and held six 100ml cylindrical tubes (each with conical ends) in a fixed position within a 4000ml cylindrical bottle. All structural components were plastic. Figure 1 shows sagittal, coronal and axial views of the phantom.
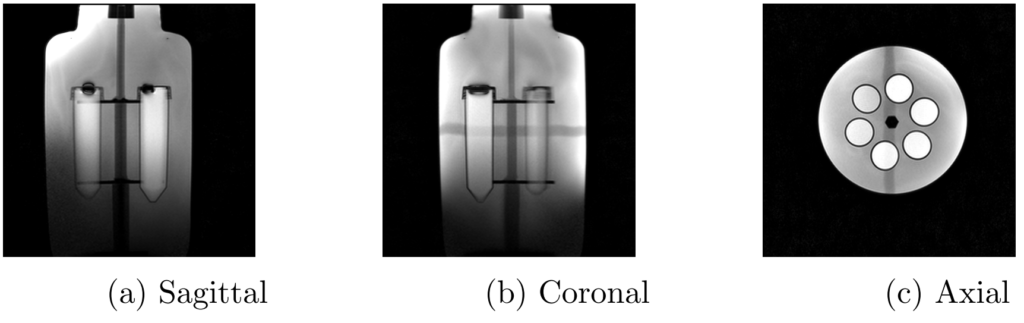
Figure 1: Three views of the custom phantom [Allen2019], from a localiser scan on a clinical Siemens scanner. A localiser scan is a quick pulse sequence used to view the internal structure and plan the positioning of subsequent image acquisitions.
Ingredients for a Range of T1 and T2
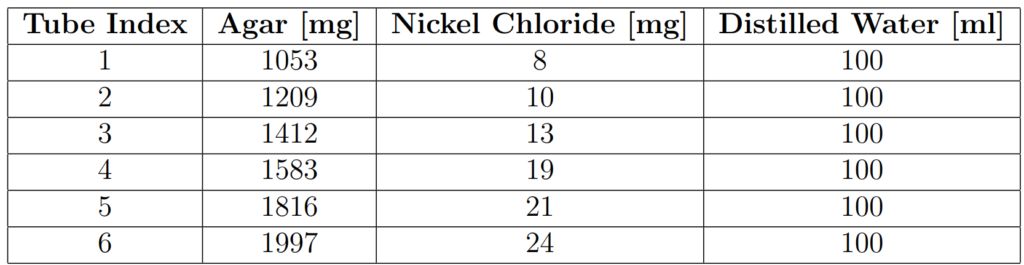
Each of the six tubes contained solid agar gel doped with Nickel Chloride (NiCl), in varying concentrations such that each tube had different MR relaxation properties. The concentrations in [Zhu2005] were used as a guide for the approximate concentrations needed to achieve the desired T1 and T2 range. The gels were formed by mixing powdered agar and Nickel Chloride with distilled water. The 4000ml bottle contained only distilled water.
Table 1 shows the approximate concentrations of agar and Nickel Chloride used for each tube.
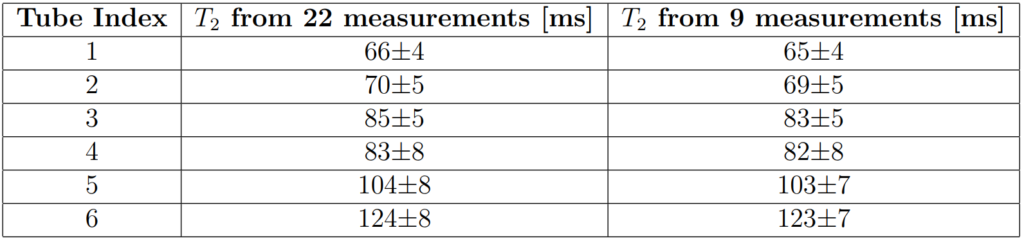
The T1 and T2 of the phantom tubes were characterised using reference measurement methods, showing that T1 varied across the tubes from approximately 840ms to 1640ms (Table 2) and T2 varied from approximately 60ms to 130ms (Table 3). The T2 measurements can also be seen in this post: Implementing a variable echo time method for reference T2-mapping.
Temperature
Relaxation parameters T1 and T2 depend on temperature [Statton2022]. The T1 and T2 values in Tables 2 and 3 were measured at scanner room temperature, which was approximately maintained for all subsequent acquisitions in [Allen2019]. Assuming the temperature is relatively consistent, reference T1 and T2 measurements are valid for subsequent experimental measurement methods and comparisons. Measured T1 and T2 may be corrected to account for temperature differences [Statton2022].
References & Further Reading
- J. Allen, ‘An Optimisation Framework for Magnetic Resonance Fingerprinting’. PhD thesis. University of Oxford. 2019. https://ora.ox.ac.uk/objects/uuid:14c92874-7b00-4f79-abce-87b05f9cb4d4
- M. Cercignani, N. G. Dowell, and P. S. Tofts. Quantitative MRI of the Brain. Wiley Online Library, 2nd edition, 2018.
- J. Z. Bojorquez, S. Bricq, C. Acquitter, F. Brunotte, P. M. Walker, and A. Lalande. What are normal relaxation times of tissues at 3 Tesla? Magnetic Resonance Imaging, 35:69–80, 2017.
- R. C. Brand, N. P. Blockley, M. Chappell, and P. Jezzard. Clinically relevant rapid 3D CEST imaging with hexagonal spoiling gradients, optimised B1, and symmetric z-spectrum sampling. In Proceedings of the 24th Annual Meeting of ISMRM, page 299, 2016.
- D. C. Zhu and R. D. Penn. Full-brain T1 mapping through inversion recovery fast spin echo imaging with time-efficient slice ordering. Magnetic Resonance in Medicine, 54(3):725–731, 2005.
- B. K. Statton, J. Smith, M. E. Finnegan, G. Koerzdoerfer, R. A. Quest, and M. Grech‐Sollars, “Temperature dependence, accuracy, and repeatability of T1 and T2 relaxation times for the ISMRM/NIST system phantom measured using MR fingerprinting,” Magnetic Resonance in Med, vol. 87, no. 3, pp. 1446–1460, Mar. 2022, doi: 10.1002/mrm.29065.